Leverage the power of PlacebellⓇ
The only placebo effect quantification tool to optimize clinical trial success.
Not managing the placebo response puts your clinical trials at risk -
a risk you can now avoid. Take action today.
Quantify the power of the mind.
To de-risk your phase ll and lll clinical trials starting today.
Every clinical trial patient is unique - and so is their placebo response. That's why high failure rates in Phases II and III are still too common. How are you accounting for the placebo response in your clinical trials?
Placebell offers a sophisticated, yet simple, AI-based method to predict and account for individual patients' placebo responsiveness in clinical trials. Deployed with only minimal changes to protocols and with no risk to your data analysis, Placebell helps sponsors bring life-saving therapies to market safely and efficiently.
0%
Increase in study power0%
Decrease in variability0%
Reduction of type ll errorPrediction of individual patient placebo responsiveness
The examples below present in a simple way (placebo responders versus non-responders) the accuracy of this prediction. When in the examples, PlacebellⓇ is presented as binary (placebo responders versus non-responders), in reality, PlacebellⓇ is a continuous score to be used as a baseline prognostic covariate to improve the study power.
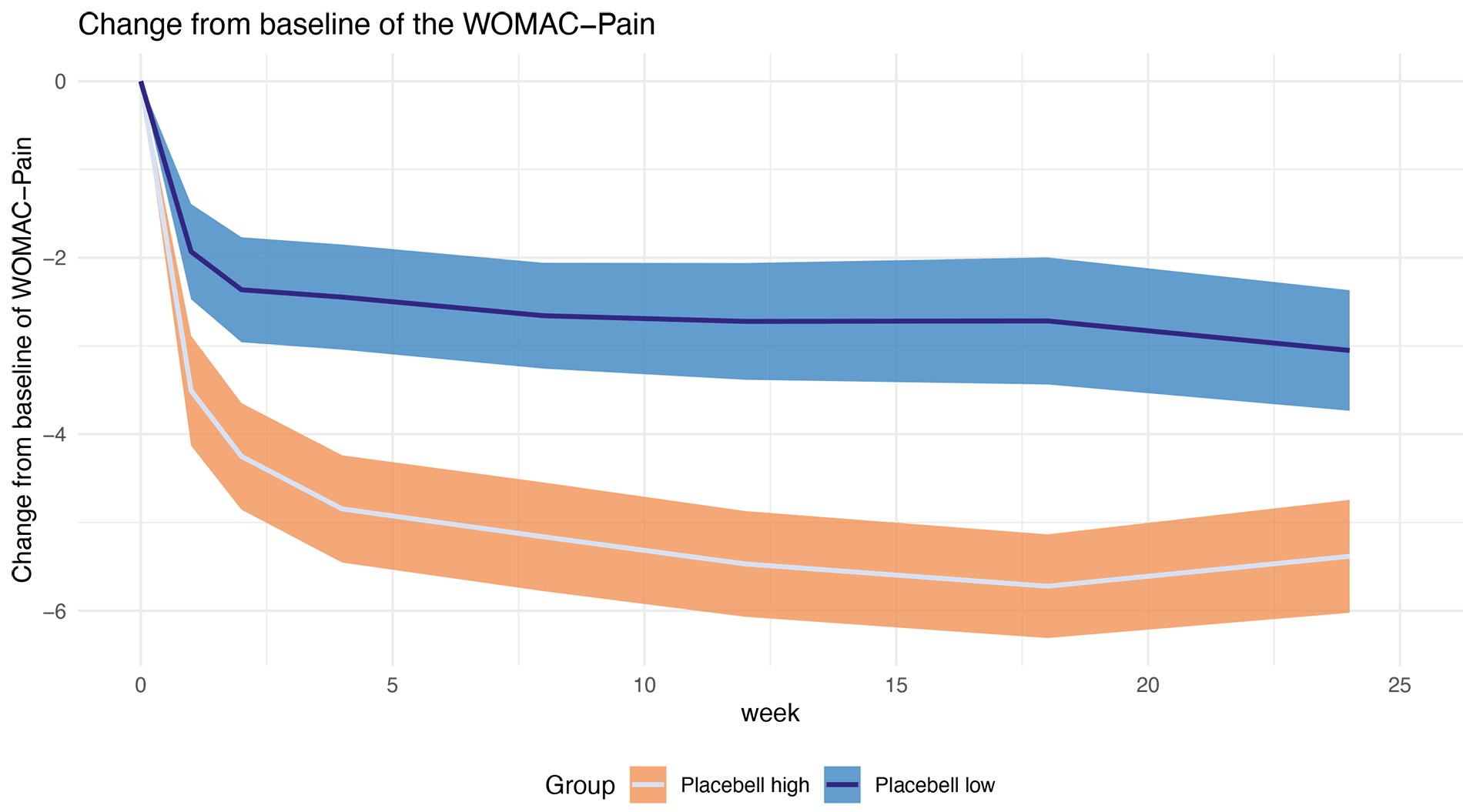
Example of a subjective endpoint-Prediction of Womac pain improvement in osteoarthritis: Placebell can predict the placebo responders (Placebell high) who will have a higher pain decrease and non-responders (Placebell low) with a lower pain decrease. This prediction is highly statistically significant
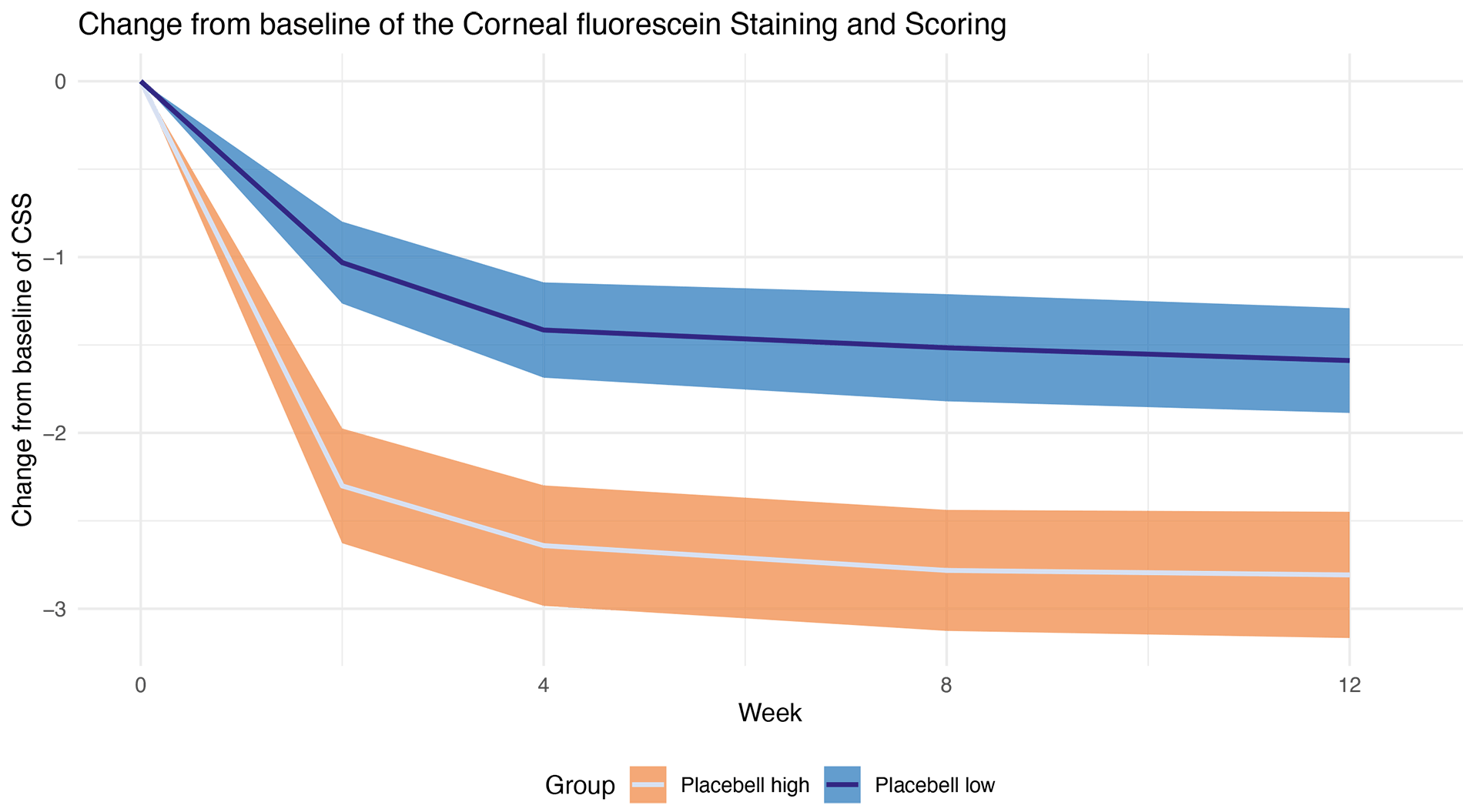
Example of an objective endpoint-Prediction of the Corneal Fluorescein Staining and Scoring (CFSS) in dry eyes: Placebell can predict the placebo responders (Placebell high) who will elicit a higher CFSS decrease and non-responders (Placebell low) with a lower CFSS decrease. This prediction is highly statistically significant.
proven
Placebell has been deployed in more than a dozen studies across multiple indications.
low risk
Placebell poses no mathematical or operational negative impact on the trial or data.
powerful
Placebell uses advanced AI and machine learning to reduce variability in efficacy evaluation.
Step 1 Assess patient psychological profile
Patients are administered a validated questionnaire only once in a trial. This measures personality traits, expectations and other factors.
Step 2 Calculate placebo response through our AI-powered platform
The PlacebellⓇ Covariate - defining each patient's relative placebo responsiveness - is calculated using a machine learning-based model.
Step 3 Define and use covariates to de-risk trials
The Placebell©TM Covariate can then be used in statistical analysis to decrease variance and increase study power.
The next frontier in clinical research & patient management
We’re proud to be leading the charge into the next era of drug development.
Cognivia helps clinical trials reduce data variability, empower decision-making, and accelerate the launch of new therapies.
Tell us about your clinical trial below and we’ll be in touch.
"*" indicates required fields
